FDA clears new energy-efficient MRI scanner for human and veterinary use
The Magnetom Flow.Ace also uses AI technology for faster scans and image reconstruction.
The FDA has cleared a 1.5 tesla (T) platform for magnetic resonance imaging (MRI)—the Magnetom Flow.Ace—by Siemens Healthineers, a global provider of health care equipment. The system features a closed helium circuit and no quench pipe that significantly reduces its reliance on helium, according to Siemens.1
The Magnetom Flow.Ace is the company’s first MR scanner designed for veterinary radiology and general radiology markets. It features a 60-cm bore and covers a comprehensive range of MR applications.
According to Siemens, the system has image reconstruction that uses artificial intelligence (AI) to provide faster scans with improved image quality. Specifically, the AI allows for reduced exam times. Moreover, the tool features Deep Resolve image reconstruction technology that allows for faster image acquisition, eliminates noise, and improves the acuity of small structures.
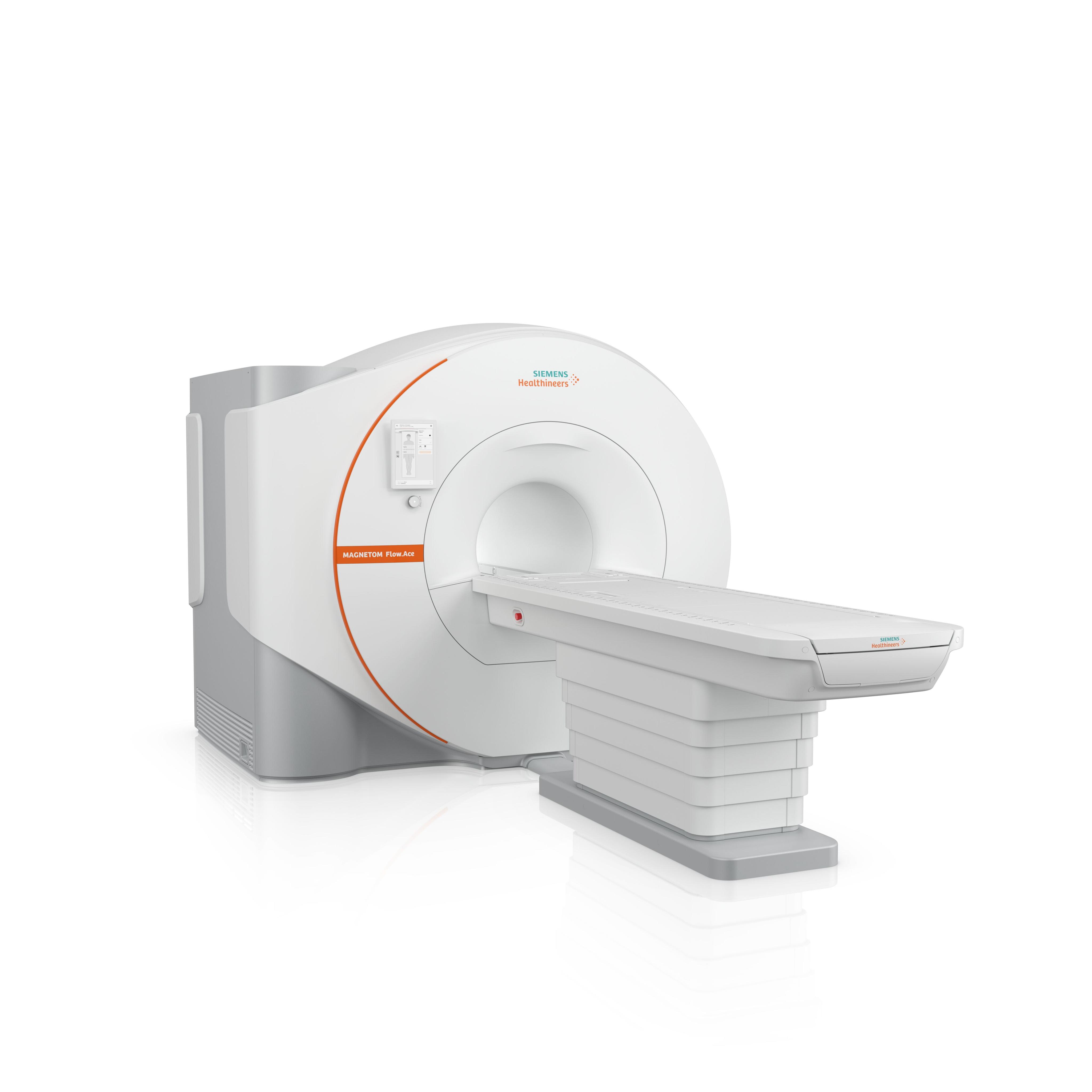
The product is the company’s second MR platform that is “virtually” helium free, after its 0.55T Magnetom Free platform.1 According to Siemens, the new system only needs 0.7 L of liquid helium for cooling. In contrast, traditional MRI scanners generally require more than 1000 L of liquid helium.1
The reduced amount of helium needed for the Magnetom Flow.Ace platform can be attributed to its magnets, which use DryCool technology that reduces its dependency on helium refills, consequently saving resources and lowering costs. The system also does not need a quench pipe, a feature that was traditionally necessary to safely vent large volumes of gaseous helium into the atmosphere during emergency shutdowns. “This lack of a quench pipe, combined with the scanner’s compact size, can reduce installation requirements and costs compared with those of other 1.5T MR scanners,” Siemens stated in a company release.1
“With the FDA clearance of the Magnetom Flow.Ace, Siemens Healthineers is pleased to introduce our first helium-free 1.5T MR scanner,” Katie Grant, head of magnetic resonance at Siemens Healthineers North America, said in the release.1 “This 60-cm bore system is also available for veterinarians, with the same intelligent, high-end technology that we’ve offered to the general imaging community, at a highly attractive total cost of ownership.”
The product contains a variety of contour and dedicated MR coils that can be used for human anatomies, alongside coils that can be used in veterinary settings. “It's animal-friendly, high-density contour coils, which are available in [3] sizes, are flexible and adaptable to different anatomies,” Siemens stated.1 The coils feature an integrated BioMatrix Position Sensor that facilitates automated patient positioning for “potentially” quicker exam setup. A complete range of specialized imaging coils is also offered, including those designed for the brain and spine.
According to Siemens, the new product uses more than 30% less energy each year compared with the company’s older MR scanners. It also has an Eco Power Mode feature that automatically turns off energy-intensive parts when they are not in use.
Reference
- Siemens Healthineers receives FDA clearance for Magnetom Flow.Ace, company’s first helium-free 1.5 tesla magnetic resonance scanner. News release. Siemens Healthineers. June 26, 2025. Accessed June 26, 2025. https://www.siemens-healthineers.com/en-us/press-room/press-releases/magnetom-flow-ace-fda-clearance